
Gennova is dedicated to the development and production of bio-therapeutics to address life-threatening diseases across various areas. Gennova has multiple collaborations with research institutes and organizations across the globe which have helped build its expertise.

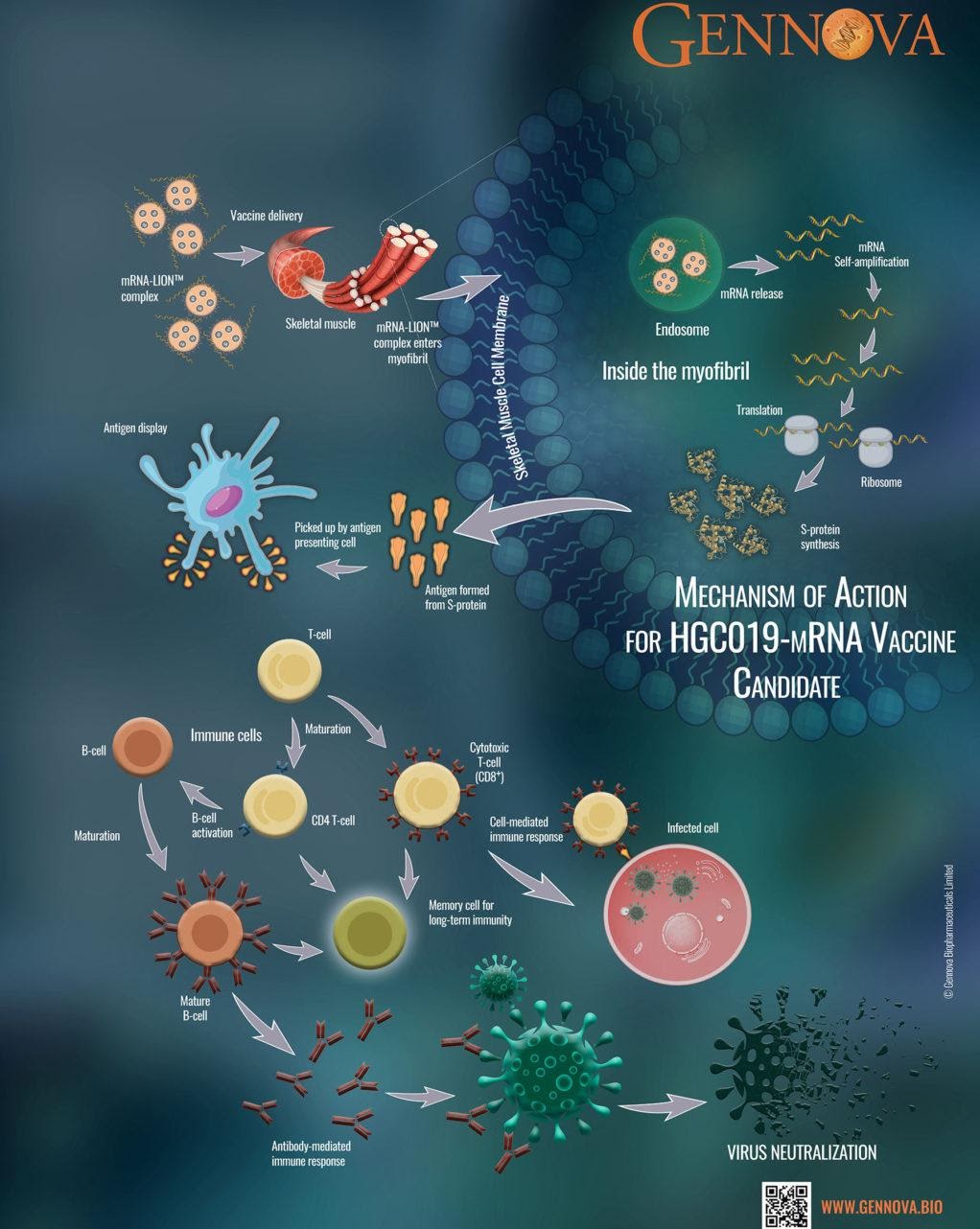
Over the past few years, Gennova has been working on developing an mRNA platform for developing bio-therapeutics and vaccines. It currently has four vaccines in its pipeline including India’s first indigenously developed mRNA vaccine, HGC019 which is in Phase I clinical trials. Gennova is also leveraging this technology to develop vaccines for Zika, Rabies and Zoster which are in the pre- clinical stage.
What are mRNA vaccines?
In a standard viral vaccine, either inactivated (or attenuated) virus or viral proteins known to cause the infection are used for immunization. However, an mRNA vaccine carries the molecular instructions to make the protein in the body through a synthetic RNA of the virus. The host body uses this to produce the viral protein that is recognized by the immune system, thereby making the body ready to fight against the disease.

Biologics Research & Development
Emcure Pharmaceuticals is committed to delivering high- quality biologics that are affordable. It is also in the process of developing new delivery systems which allow ease of use. Gennova has worked on a platform strategy with a focus on developing platforms and then using these to launch multiple products. It currently has two established biologics platforms – Mammalian and Microbial.
Gennova has concentrated its research and development efforts to create specifically designed mammalian production cell lines that can survive more than 90 days in a perfusion-based bioreactor system. This innovative approach has allowed Gennova to not only commercialize these life-saving bio-therapeutics at an affordable price but also achieve high-quality & uniformity across batches which is a key challenge in biologic production. Gennova is also leveraging AI tools to improve manufacturing processes, quality and yields of products. It also intends to employ Machine Learning Algorithms to develop next-gen, data driven automated bio-manufacturing processes to minimize manufacturing deviations and meet regulatory compliance and improve cost efficiencies.
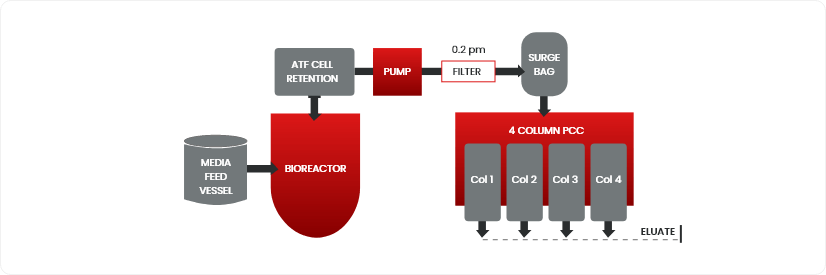
Continuous Bio-manufacturing:
| Product | Benefit |
| Tenecteplase for AMI |
|
| Tenecteplase for AIS |
|
Gennova’s innovation around high cell density fermentation, genetic manipulations for its microbial products and a cost-effective pegylation technology puts it in a unique position to address the problems of quality, affordability and availability of the portfolio of products.
The key innovations include:
| Product | Benefit |
| Pegaspargase: Hamsyl Commercial |
|
| Recombinant Asparaginase: Pipeline |
|